Abstract
INTRODUCTION: Salvage chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard of care in the management of relapsed or refractory (R/R) classical Hodgkin lymphoma (HL). Historically, salvage chemotherapy regimens such as ICE (Ifosfamide, carboplatin, and etoposide) and GDP (gemcitabine, dexamethasone, cisplatin) have been used. However, newer regimens such as bendamustine with brentuximab-vedotin (BBV), and BV with nivolumab are being increasingly used owing to their improved efficacy. The impact of the newer regimens on subsequent mobilization and collection of peripheral blood stem cells (PBSCs) for ASCT is not studied in detail. Furthermore, despite its well reported use, the mobilizing agent "plerixafor", which is approved for multiple myeloma and non-Hodgkin lymphoma, is not FDA approved for HL. This creates a logistic barrier in using it in HL patients.
METHODS: We conducted a retrospective chart review of patients with R/R HL referred to Karmanos Cancer Institute and considered for ASCT between January 1, 2015, and December 31, 2021. Patients were grouped in two groups based on the pre-ASCT salvage chemotherapy regimen used: ICE/GDP and BBV. We analyzed multiple parameters to study the impact of ICE/GDP versus BBV on mobilization/collection of PBSCs for ASCT in R/R HL. We also describe our institutional experience with the usage of plerixafor in addition to G-CSF for PBSC mobilization.
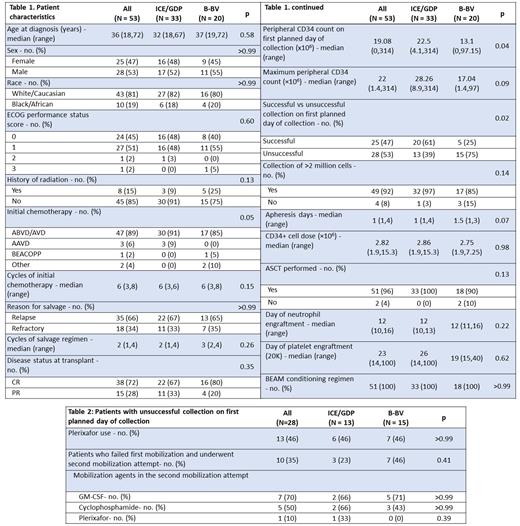
RESULTS: A total of 53 patients with R/R HL who underwent mobilization for ASCT. The salvage regimen was ICE/GDP in 33 (62%) and BBV in 20 (38%) patients. There was no difference in the median age at diagnosis (32 vs 37 years, p=0.59), and distribution of sex (p>0.99), race (p>0.99) and ECOG performance status (p=0.60) (See Table 1). Majority of the patients received ABVD/AVD (Adriamycin, bleomycin, vinblastine, dacarbazine) as their initial treatment (ICE/GDP 91% vs BBV 85%, p=0.06), with a median of 6 cycles (p=0.16), and no difference in the use of radiation therapy (p=0.14). Thereafter, around two-third of the patients underwent salvage chemotherapy for relapsed, and one-third for refractory disease in both arms. The median number of cycles was 2 (1-4) versus 3 (2,4) (p=0.267), and the CR rate was 67% versus 80% in ICE/GDP and BBV respectively (p=0.36). Stem cell mobilization was performed using G-CSF and median peripheral CD34 count (×106) on day 1 of collection was higher at 22.5 (range, 4.12-314) in the ICE/GDP group versus 13.1 (0-97.15) in the BBV group (p=0.049). Twenty (61%) patients in the ICE/GDP had a successful stem cell harvest following G-CSF (>2 million cells/kg) compared to 5 (25%) in the BBV group on first planned day of collection (p=0.02).
Among the patients who failed successful stem cell collection with G-CSF on the first day of collection, 6 (46%) received plerixafor in the ICE/GDP and 7 (46%) in the BBV group (See Table 2). Notably, 3 (23%) patients in the ICE/GDP whereas 7 (46%) patients in the BBV group failed the first attempt of mobilization and had to undergo a separate second attempt with GM-CSF, cyclophosphamide and/or plerixafor. The cumulative number of mobilizing agents other than filgrastim used in any attempt was 11 (0.3 per patient) and 15 (0.75 per patient) in ICE/GDP and BBV groups respectively. Furthermore, 2 patients in the BBV group had a failure of adequate mobilization/collection of PBSCs despite more than one attempt, and were unable to proceed with ASCT. One of these patients died of progressive HL. In contrast, all the patients were able to undergo ASCT in ICE/GDP group. There was no difference in the day of neutrophil or platelet engraftment between the two groups.
CONCLUSIONS: We conclude that BBV regimen led to poor mobilization and collection of stem cells on the first day, consequently requiring additional mobilization agents/attempts. Although, PBSC collection and subsequent ASCT was eventually successful in a majority of the patients in the BBV group, two patients in our population failed to undergo ASCT given inadequate mobilization/collection. With the increasing utilization of brentuximab-vedotin in salvage as well as in the first line setting, larger studies are needed to study its impact on PBSC collection and ASCT.
Disclosures
Modi:AstraZeneca: Honoraria; Karyopharm Therapeutics: Research Funding; Beigene: Speakers Bureau; ADC Therapeutics: Research Funding; Genentech: Research Funding; Seagen Inc.: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Membership on an entity's Board of Directors or advisory committees. Deol:Janssen: Consultancy; Kite, a Gilead Company: Consultancy; Adicet: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal